


1.Patient Profile
The patient, a 7-year-old boy, sustained a severe traumatic brain injury in a traffic accident in November 2023. He underwent epidural hematoma evacuation and decompressive craniectomy at a local hospital, followed by intracranial pressure–lowering and symptomatic supportive therapy. Despite surgery, the patient remained comatose. In January 2024, follow-up CT revealed communicating hydrocephalus. A ventriculoperitoneal (VP) shunt was performed in our hospital, stabilizing his condition. Subsequent neurorehabilitation at another institution left him in a semi-conscious state with increased muscle tone in all limbs and loss of self-care ability. For further management of cranial defect, post-traumatic recovery, and post-shunt hydrocephalus, he was admitted to the Department of Neurosurgery, Guangdong Second Provincial General Hospital, on May 15, 2024
1.2.Examination
Right fronto-temporo-parietal cranial defect, approximately 14 × 16 cm, with sunken decompression window.
Neurological status: comatose with eye-opening only. Tracheostomy in place. Pupils equal and reactive (2.0 mm), free ocular movements. Increased limb muscle tone, motor strength grade 2/5. Physiological reflexes present, no pathological reflexes elicited.
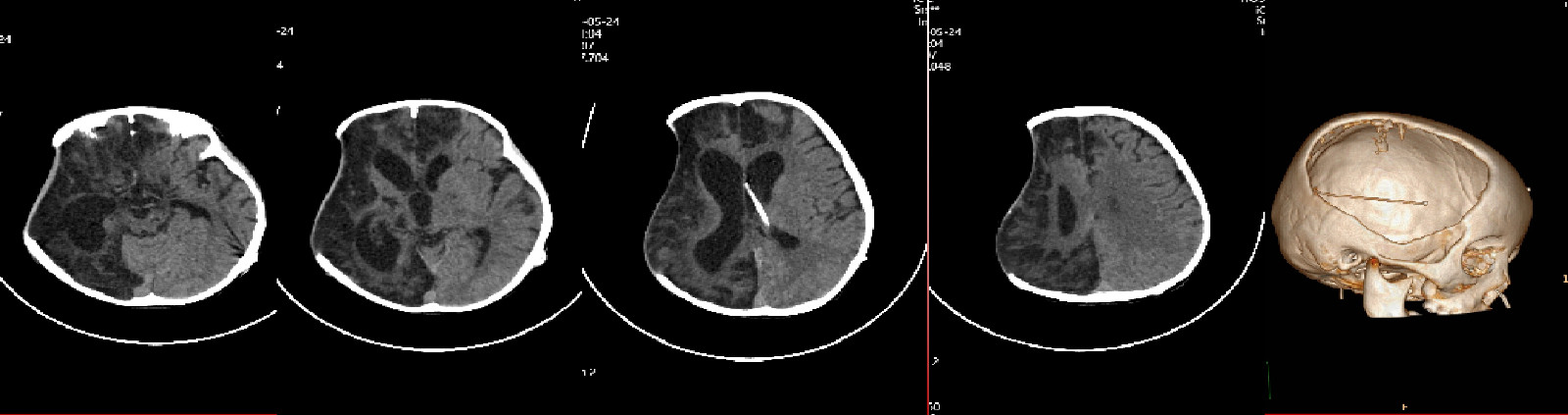
FIGURE 1:Preoperative Scan
2.Treatment Plan
2.1.Preoperative Considerations
As a 7-year-old child, cranial growth must be considered. Repair material should be customized with a 1–2 mm margin from the cranial edge.
Presence of VP shunt and markedly sunken bone window increase the risk of postoperative hematoma/effusion beneath the implant. The shunt valve pressure was set to 150 cmH₂O preoperatively to allow brain re-expansion, then gradually lowered to 110 cmH₂O on postoperative day 5.
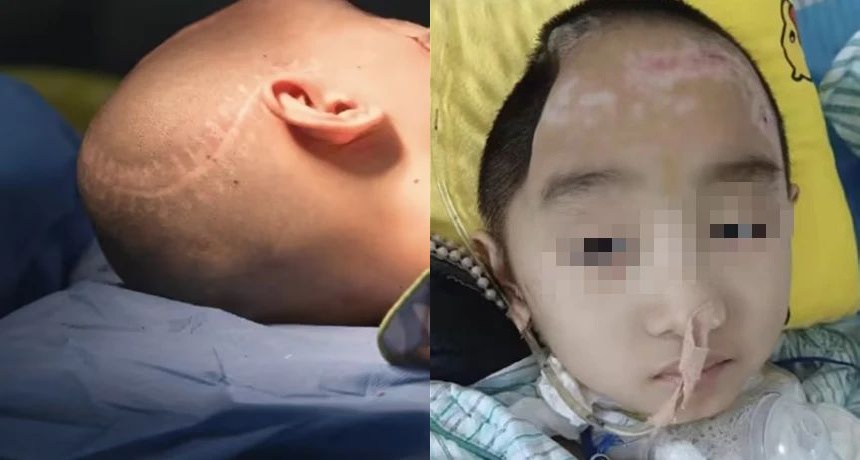
FIGURE 2:Preoperative condition
2.2.Surgical Procedure
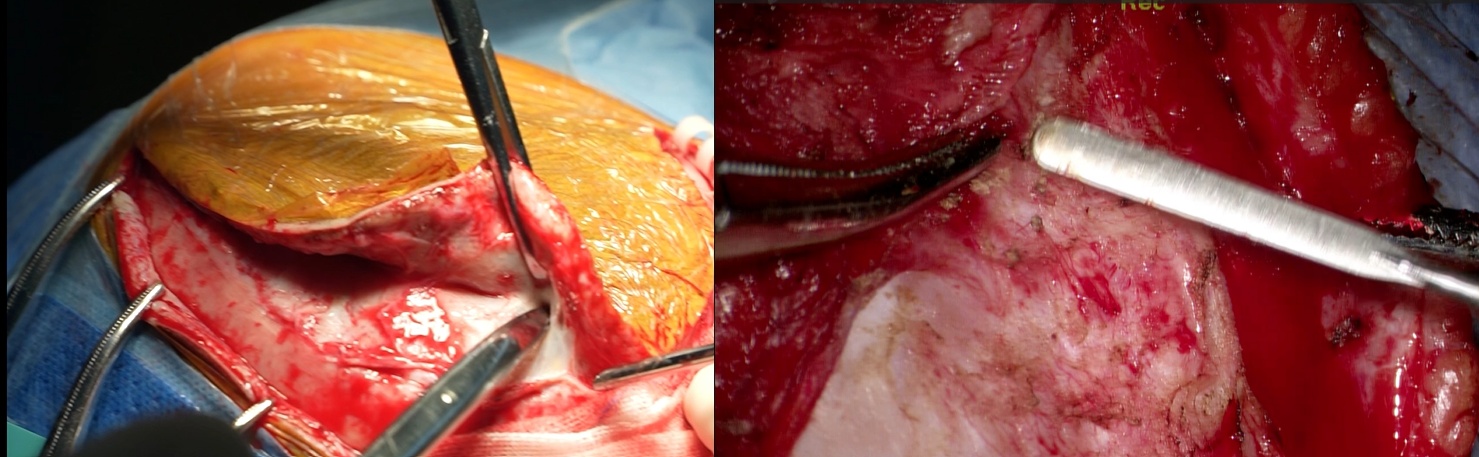
FIGURE 3:Surgical Procedure Step 1-Scalp, galea, and periosteum separated; soft tissue cleared from bone window margins to expose the defect.
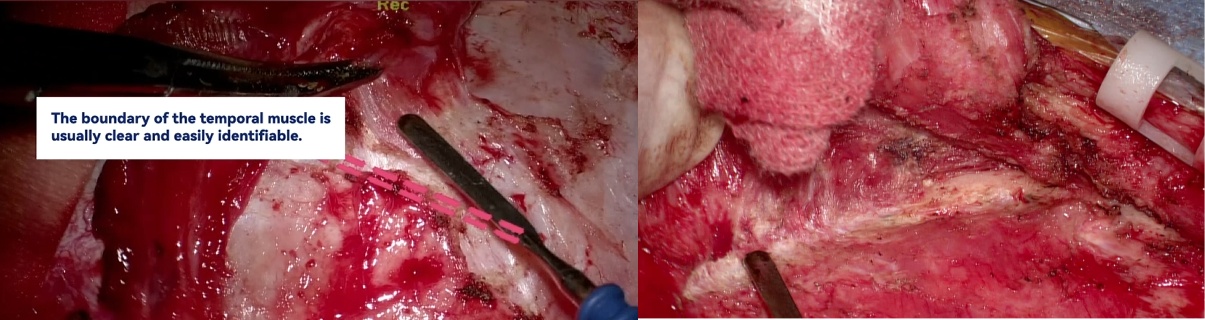
FIGURE 4:Surgical Procedure Step 2- Temporal muscle detached and reflected to fully expose bone window.
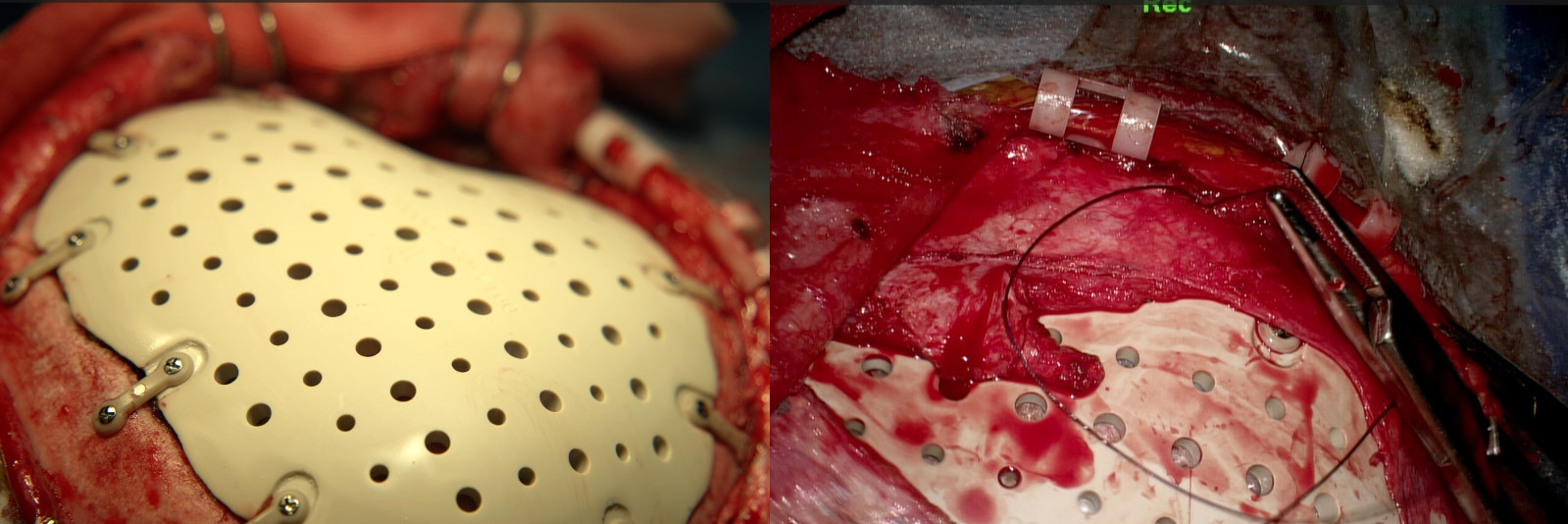
FIGURE 5:Surgical Procedure Step 3- Customized PEEK (polyetheretherketone) cranial implant installed and secured with PEEK fixation plates; periosteum and temporal muscle flap sutured.

FIGURE 6:Surgical Procedure Step 4- Drainage tube placed, meticulous hemostasis achieved, scalp closure performed.
3.Postoperative Outcomes-
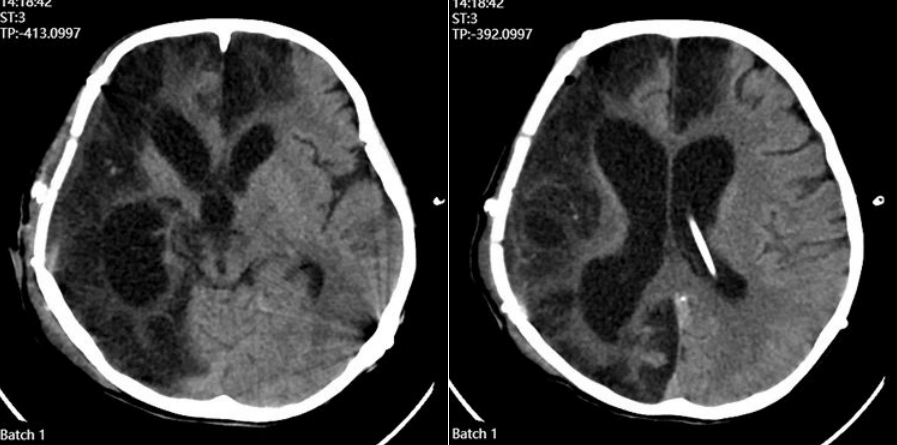
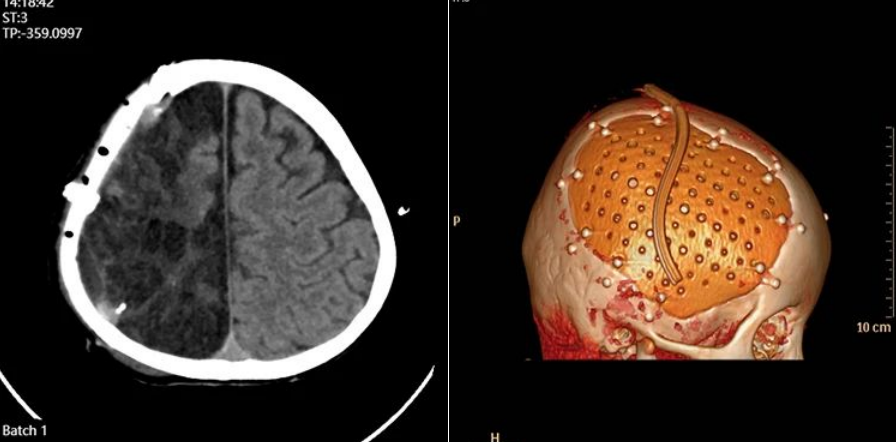
FIGURE 7:Postoperative day 1
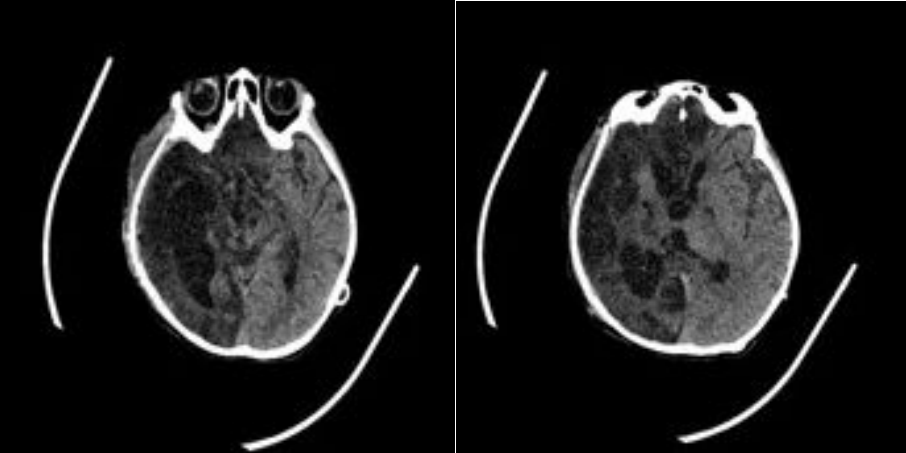
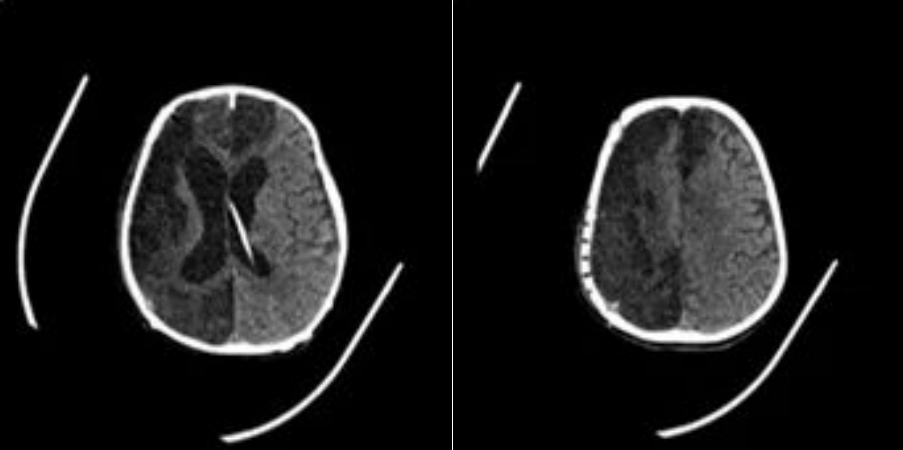
FIGURE 8:Postoperative week 1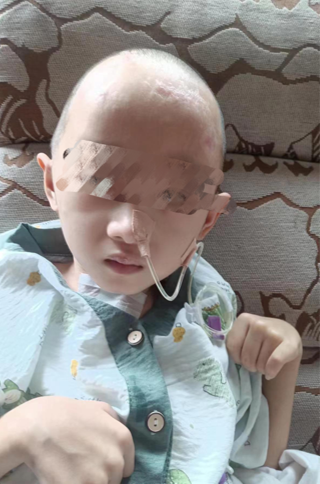
FIGURE 9:Postoperative Month 1
4.Case Discussion-Optimizing Outcomes in Cranioplasty: Key Preventive Measures
Cranioplasty is a common neurosurgical procedure, broadly categorized as a reconstructive surgery. Patients generally have high cosmetic and functional expectations. However, complications such as infection, hemorrhage, and implant exposure often necessitate removal of the prosthesis, leading to repeated trauma and potential medico-legal disputes.
To optimize outcomes and reduce complications, the following measures are critical:
Strict aseptic technique – perioperative and intraoperative infection control.
Protection of wound vascularization – preservation of scalp blood supply.
Prevention of subdural effusion – careful intraoperative handling and postoperative drainage.
4.1.Strict Aseptic Technique
Preoperative:
Scalp care is essential, particularly for long-term bedridden patients.
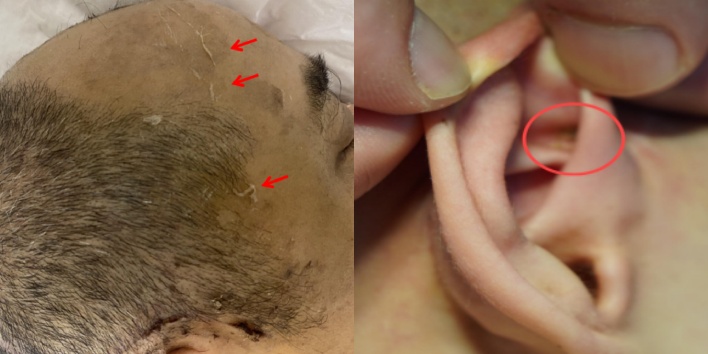
FIGURE 9:Example: visibly poor scalp hygiene compared to facial skin; debris concealed in auricular folds.
Intraoperative:
(1)Complete scalp disinfection and iodine drape coverage.
(2)Wound bed irrigated repeatedly with povidone-iodine, hydrogen peroxide, and saline prior to implant placement.
(3)Anatomical restoration (e.g., separating “periosteum–temporal muscle flap”) to enhance anti-infection capacity.
(4)Ensure frontal sinus and mastoid air cells are not opened; avoid stripping soft tissue over pneumatized areas.
Postoperative:
After suture removal, patients are encouraged to wash hair on day 3 to maintain scalp cleanliness.
4.2.Protection of Wound Blood Supply
(1)Re-incision strictly along original scar line.
(2)Minimize scalp clip and electrocautery use at wound edge.
(3)Preserve superficial temporal artery when incising preauricular area.
(4)Intradermal suturing recommended for scalp closure.
4.3.Prevention of Subdural Effusion
(1)Careful assessment of wound healing and nutritional status preoperatively.
(2)Minimize electrocautery injury during surgery.
(3)Avoid excessive flap traction that impairs venous return.
(4)Gentle dural dissection to avoid tearing.
(5)Preserve periosteum at bone edges to reduce oozing.
(6)Dural tears should be promptly repaired; adjunctive dura sealant, muscle/fascia grafts, and surgical glue may be used.
(7)Subgaleal drains with negative-pressure suction and compressive dressings are recommended when necessary.
(8)Dura suspension on PEEK implant is not recommended. Sunken dura results from atmospheric pressure and restores slowly; suspension increases risk of CSF leakage and subgaleal effusion.
5.Additional Remarks
Optimal cranioplasty begins not at reconstruction but during the initial decompressive craniectomy — e.g., handling of temporal muscle and use of artificial dura can reduce postoperative muscle atrophy and simplify future reconstruction.
Role of PEEK in Cranioplasty:
PEEK is an ideal material for cranial repair, offering biocompatibility, strength, and excellent cosmetic results. With standardized perioperative management, its advantages can be fully realized, reducing complications and enabling durable, patient-specific reconstruction.






