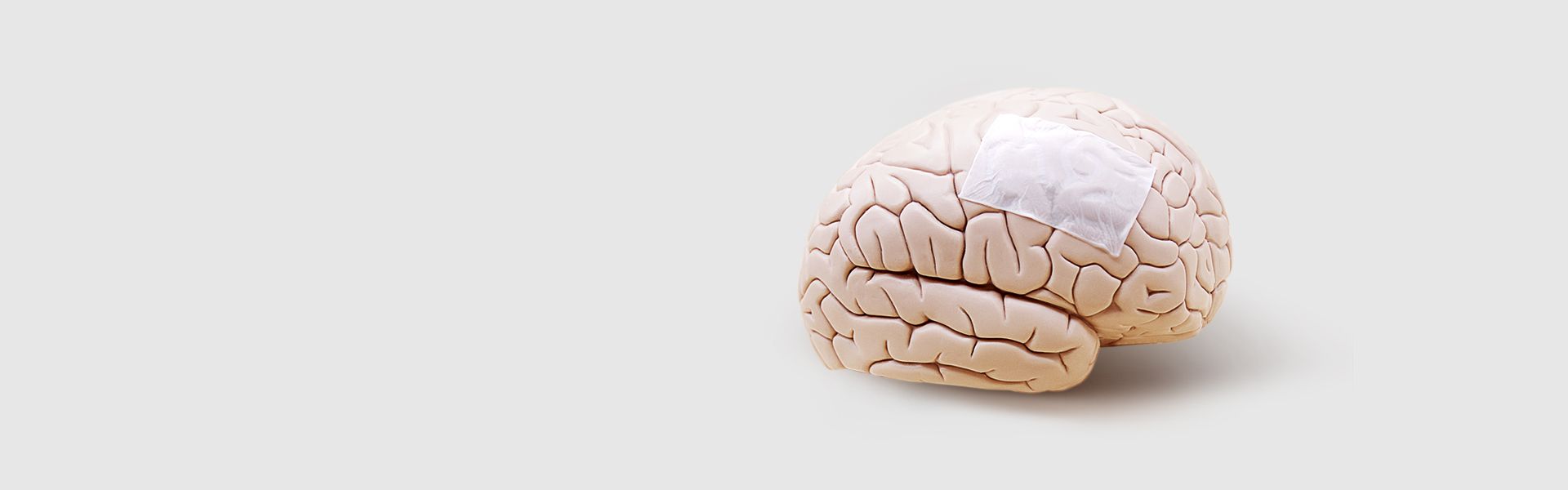
ReDura™ Synthetic Dural Substitute
FDA approved degradable material poly-L-lactic acid (PLA) Widely applied in 60+ countries. Outstanding efficacy and safety for the dural defect repair
Biomimetic
Similar to native extracellular matrix (ECM), rapid repair and regeneration.
Patent Technology
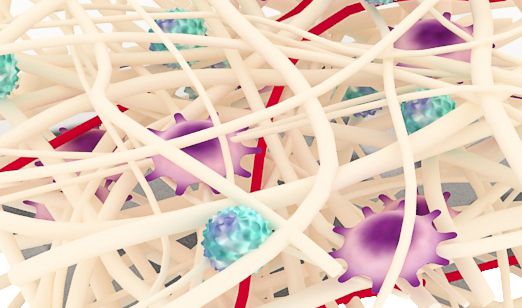
ReDura™ Mimic ECM Structure
Non-animal materials
Biomimetic-Synthetic-Absorbable Dural Substitute without increased risk of animal infection
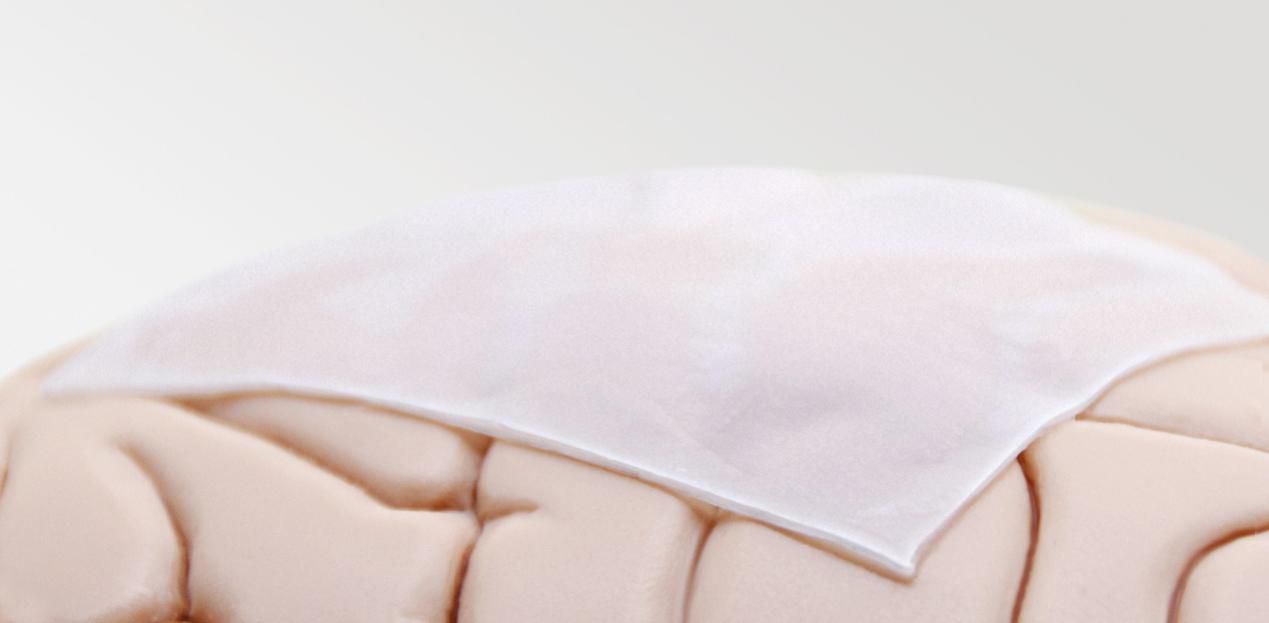
Onlay or Suture
Depends on surgeon habit with flexibility
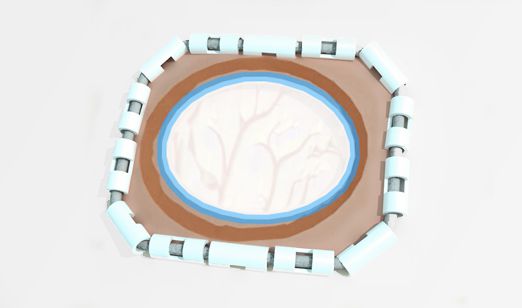
Onlay
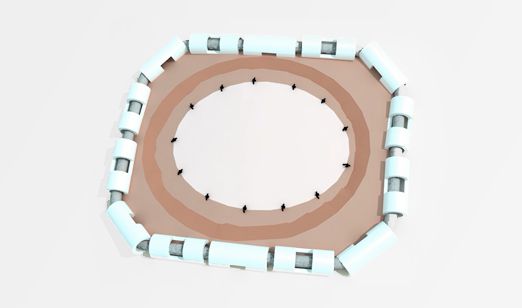
Suture
