
StypCel™Absorbable Hemostat
100% absorbable within 7-14 days. Hemostasis in capillary, venous as well as small artery bleeding. StypCel™ works when ligation or other conventional methods are impracticable or ineffective
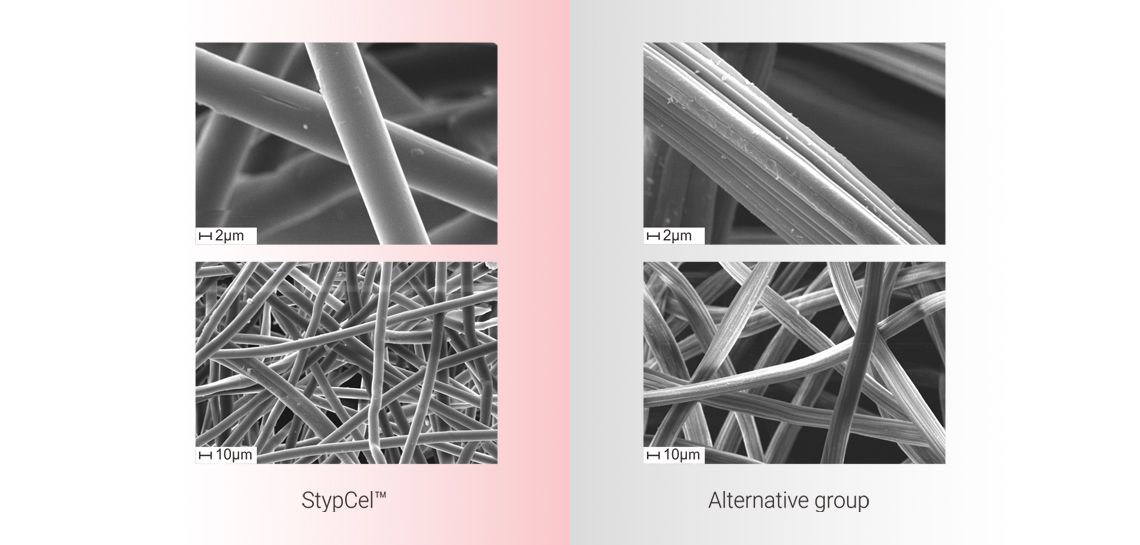
Slender fibers
more compact structure
provides StypCel™ larger contact area attaching to wound surface so as to strengthen hemostatic effect
Easy peeling
Simple shaping

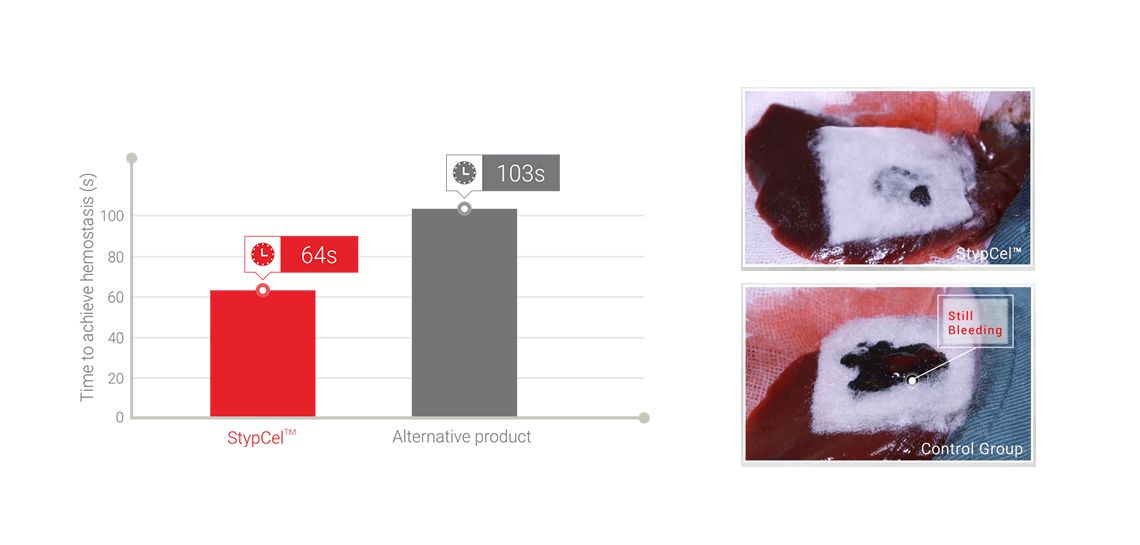
Fast hemostatic rate
pre-clinical study on livers of rabbits, result demonstrates StypCel™ hemostatic faster
IFU
LABEL
Document Name
Status
Publication Date
You may have an access to the SSCP by sending an email to information@medprin.com, before the SSCP module of EUDAMED is functional.
